This colorful image shows the different colors that we can see in an aurora. This year 2024 it’s supposed to be the best season since the last 20 years to see active auroras all around the northern and southern polar regions. Knowing that it could be a good chance to capture images of this natural phenomena I travelled to Norway, for the second time in the last 3 months, in order to see the aurora activity. I spent 4 nights guiding a group, from the 1st to the 5th of February 2024. The weather was harsh and we just arrived after a storm called Ingunn by the Norwegian meteorologists that obliged part of our group to arrive one day lanter, on the 2nd of February due to the partial closure of Tromsø airport.
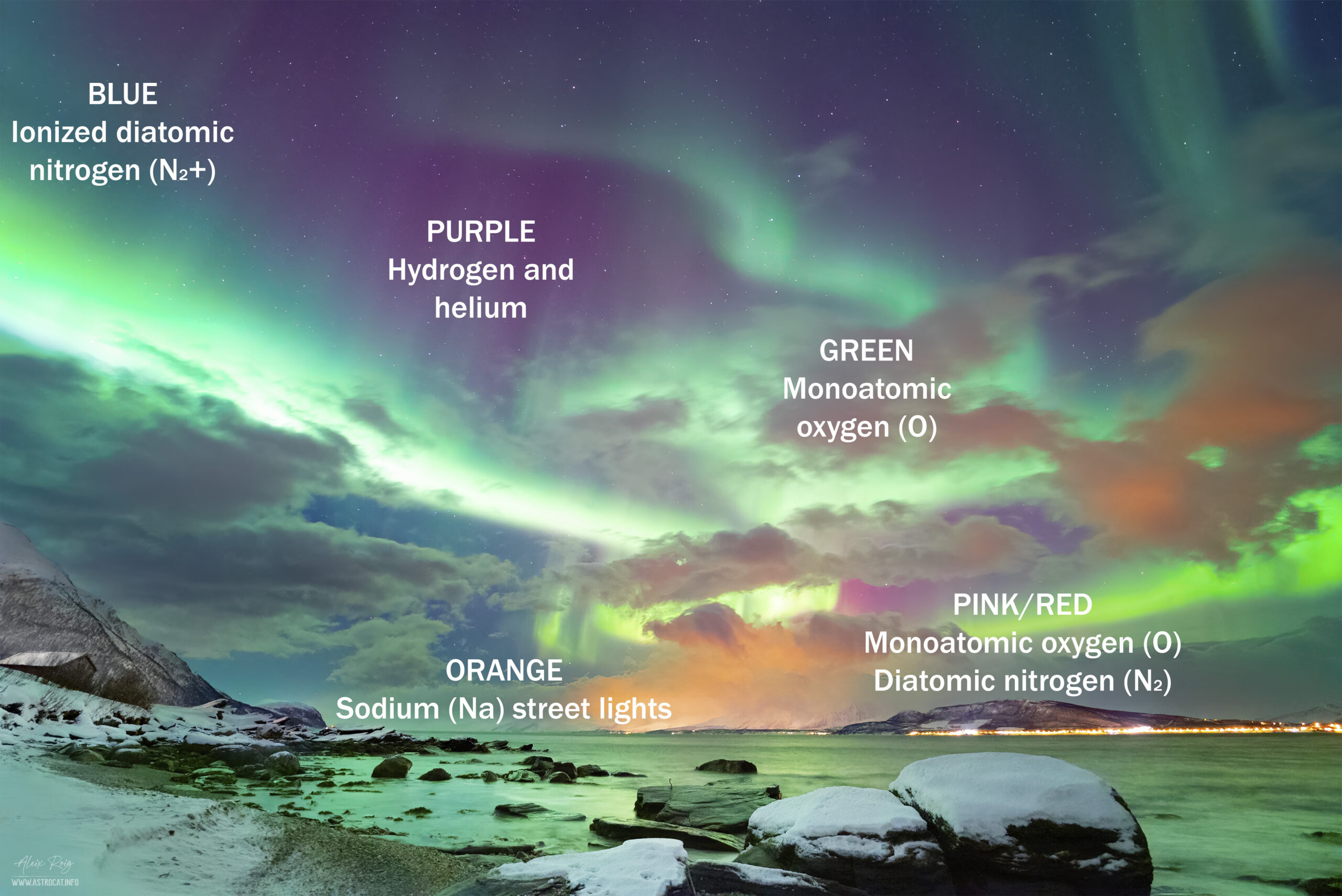
The last night of the trip a high aurora activity was predicted and we searched for a clear sky in the middle of the cloudy weather. According to the maps, there was a nice spot just NE from Tromsø that could let us see the aurora borealis. The location was Oldervik, a fishing village in the Troms og Finnmark county. Unexpectedly, when we arrived at Oldervik we could see what have been our best sights of an aurora borealis ever. The activity was high, reaching a KP5 that let us see perfectly the green colors of the aurora with the naked eye. Fast cycles of aurora pillars were followed by dancing shapes that crossed the sky from one side to another. I took my camera and, after taking several group photos, I let it work capturing a timelapse sequence. The image presented here is one single 3 second frame of this timelapse. I found it interesting as it shows a full brand of colors that are rarely seen in a lesser powerful aurora event. As well as the aurora colors shown in an annotated image, we can also see a human contribution to this colourful landscape, the street lights from the tiny fishing village of Lattervik.
The auroras are caused by the interaction of charged particles in the solar that strike the ionosphere or thermosphere layer of the Earth’s atmosphere, which contains low density gases, including nitrogen (N2) and atomic oxygen (O), as well as other elements such as hydrogen, helium and other gases. Atoms, molecules, and ions absorb the energy from these charged particles, which kicks electrons into an excited energy state. The excited state is not stable, so eventually the electrons return to a lower energy state. When this happens, the atom, molecule, or ion releases this extra energy as a photon, the light we see. The color of the light depends on the energy released by the atom or molecule. The energy that gets absorbed and the color of light released is a characteristic of that atom.
The ionosphere mainly contains nitrogen and oxygen, so these are the key players in producing the colors of the aurora. While they only release a few colors, sometimes you see a whole rainbow of hues because of the way the different wavelengths of light mix.
Thanks Science Notes for the detailed description of the aurora phenomena that documented this publication.
Thanks for your time on this website.